
Sputnik V registered in Philippines
Manila, Philippines – Russia’s Sputnik V (Gam-COVID-Vac) COVID-19 Vaccine has been approved for Emergency Use by the Philippine Food and Drug Administration.
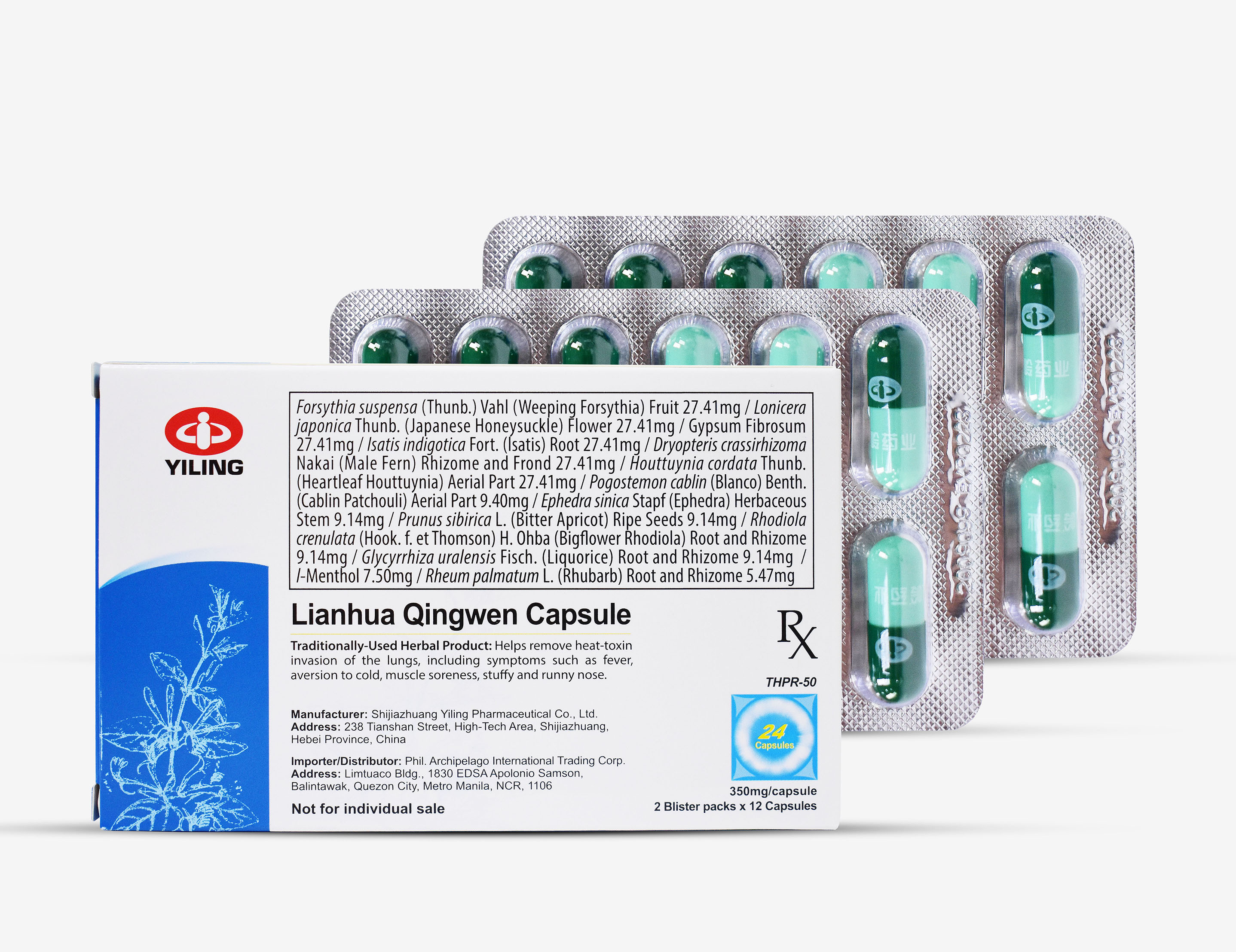
Traditionally-Used Herbal Product: helps remove heat-toxin invastion of the lungs...
Fees are an estimate only and may be more depending on your situation












Manila, Philippines – Russia’s Sputnik V (Gam-COVID-Vac) COVID-19 Vaccine has been approved for Emergency Use by the Philippine Food and Drug Administration.

The Philippines is facing a surge in coronavirus cases as it marked its first community quarantine anniversary. Due to the continuous rise of cases in the National Capital Region, the Metro Manila Council (MMC) has agreed to implement a two-week curfew which started on Monday, March 15. The executed uniform curfew hours of 10pm to 5am were consulted with the Department of Health (DOH) and independent research group OCTA experts, after being given projections if authorities will not respond to the situation urgently.

The company now sells more than 40 spirits, wines and liqueurs, including tropical-fruit blends, and exports within Asia and to the U.S. When some cities in the Philippines earlier this year banned booze amid quarantine restrictions, Destileria Limtuaco switched to making hand sanitizer and disinfectants. The fifth of seven daughters, Limpe-Aw is also president of Foresight Books Publishing & Dist. Co., Inc. and Philippine Archipelago International Trading Corporation. (Forbes Asia, 2020)
Lianhua Qingwen is a compound traditional Chinese medicine developed by exploring exogenous febrile diseases, their spread and progression laws, and their treatment based on the TCM collateral disease theory, putting forward the therapeutic strategies of "active intervention", and then formulating the therapeutic method of "clearing pestilence and detoxicating, and ventilating lung and purging heat".
Lianhua Qingwen Capsule/Granule (hereinafter referred to as LHQW) is a new Chinese patent medicine (SFDA Approval No.: Z20040063) developed under the guidance of collateral disease theory in traditional Chinese medicine (TCM) for the treatment of cold and influenza. LHQW is the first new medicine approved through fast-track approval process by the National Medical Products Administration during the SARS epidemic, has been awarded with the second prize of “National Science and Technology Progress Award”, and also become the first Chinese patent medicine that enters Phase II clinical trial approved by FDA, the US for the treatment of influenza.

Yiling Pharmaceutical Co., Ltd. announced on Monday, August 10, that it has received a drug registration certificate issued by the Food and Drug Administration (FDA) of the Philippines, indicating Lianhua Qingwen Capsule has been registered in accordance with the standards for Traditionally-used Herbal Product.